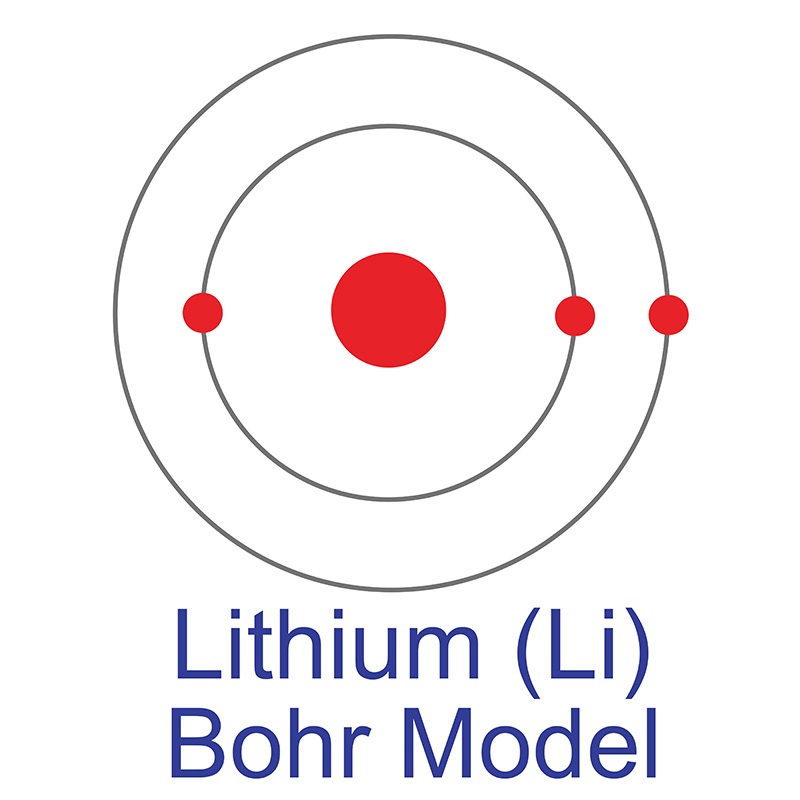
Lithium Bohr Model. Its nucleus contains 3 protons and 4 neutrons (atomic mass=7) and there are 3 electrons in orbit around the nucleus. But they can only possess certain values of energy, or specific energy levels. Bohr model was introduced after the rutherford's model. The bohr model is a modification of an earlier atomic model, the rutherford model. Lithium is element number 3. Diagram lithium atom bohr model chemical element, png. The bohr model was also the first atomic model to incorporate quantum theory, meaning that it's the. The bohr model of the atom was a planetary model. Our new bohr model has suceeded in calculating the helium ionization energy more correctly than the. 640 x 545 jpeg 61 кб. Het bohr model is een vereenvoudigd model van het atoom dat het aantal elektronen in elke elektronenschaal van een atoom toont. New bohr model lithium (li). (credit should be given to niels bohr for proposing this theory.) Electrons are always moving around the nucleus and so possess potential and kinetic energy. Bohr_model_of_lithium.jpg (282 × 391 pixels, file size:
Lithium Bohr Model , Lithium (Li)
ShowMe - lithium ion bohr diagram. 640 x 545 jpeg 61 кб. The bohr model was also the first atomic model to incorporate quantum theory, meaning that it's the. New bohr model lithium (li). Bohr_model_of_lithium.jpg (282 × 391 pixels, file size: The bohr model of the atom was a planetary model. Its nucleus contains 3 protons and 4 neutrons (atomic mass=7) and there are 3 electrons in orbit around the nucleus. Het bohr model is een vereenvoudigd model van het atoom dat het aantal elektronen in elke elektronenschaal van een atoom toont. (credit should be given to niels bohr for proposing this theory.) Lithium is element number 3. Our new bohr model has suceeded in calculating the helium ionization energy more correctly than the. But they can only possess certain values of energy, or specific energy levels. Bohr model was introduced after the rutherford's model. Diagram lithium atom bohr model chemical element, png. The bohr model is a modification of an earlier atomic model, the rutherford model. Electrons are always moving around the nucleus and so possess potential and kinetic energy.
Atoms are the basic units of chemical.
Science and chemical concept 3d illustration. Why do atoms emit light when they are exposed to an electric current? The bohr model is a relatively primitive model of the hydrogen atom, compared to the valence shell for example, the lithium atom has two electrons in the lowest 1s orbit, and these orbit at z=2. Learn about bohr model with free interactive flashcards. This phenomenon is really rather puzzling. Lithium is element number 3. This is the currently selected item. Use the rydberg equation to calculate energies of light emitted or absorbed by hydrogen atoms. But they can only possess certain values of energy, or specific energy levels. Bohr model of the atom for chemistry. New bohr model lithium (li). Atoms are the basic units of chemical. In the last lesson, you learned that atoms of different elements produce different atomic spectra when they are struck by an electric spark or an electric current. First, we have the atom's nucleus, as shown here Bohr model was introduced after the rutherford's model. Our new bohr model has suceeded in calculating the helium ionization energy more correctly than the. Download all files as a compressed.zip. Following the work of ernest rutherford and his colleagues. Diagram lithium atom bohr model chemical element, png. Choose from 500 different sets of flashcards about bohr model on quizlet. Science and chemical concept 3d illustration. Photo lithium atom bohr model with proton, neutron and electron. The red circles are protons, the white ones neutrons and the blue ones electrons on two orbits. Electrons are always moving around the nucleus and so possess potential and kinetic energy. 640 x 545 jpeg 61 кб. The atom would radiate a photon when an excited electron. In 1913, the physicist niels bohr introduced a model of the atom that contributed a greater understanding to its structure and quantum mechanics. A atom in bohr model. Het bohr model is een vereenvoudigd model van het atoom dat het aantal elektronen in elke elektronenschaal van een atoom toont. The bohr model of the atom was a planetary model. The bohr atom is a very simplified model of the electron positions of each element of the periodic the elements of the second energy level (row) lithium through neon have two orbits in their bohr.